GENE-UP®
AUTOMATED FOODBORNE PATHOGEN AND SPOILAGE DETECTION
The GENE-UP® solution is based on Real-Time PCR* technology for the detection of foodborne pathogens, spoilers and viruses in food, feed, and environmental samples.
*Polymerase Chain Reaction
?qlt=85&ts=1719873715459&dpr=off)
- Overview
- Technology
- Tests
- Services
- Resources
GENE-UP® AUGMENTED ECOSYSTEM - One Global Solution for more than 30 applications
With GENE-UP®, you can carry out over 30 testing applications, from pathogen detection for the dairy market, to virus detection in seafood, to spoilage detection for beer, wine and beverages.
With GENE-UP® , training requirements are reduced, the risk of cross-contamination is minimized, and rapid results speed up the decision-making process, improving overall production efficiency.
Main Benefits
- Ease of use and versatility
- Simplified training and workflow
- Improve lab organization, workflow and operations productivity
- Reduce cross-contamination risk
- Smoothly manage peaks of activity
- Improve reactivity in crisis management
- No need to work in batches (i.e Gram + & Gram – follow the same lysis step).
- Shorten time-to-results
Features
- Optimized enrichment media and sample preparation times
- Unique and patented lysis reagent with MAGIC CAP
- 5 minutes lysis step
- PCR run <1hour
- 2 pipetting steps only
- Specific 10uL pipetting tip
- Pre-dispensed PCR reagents
- Intuitive software
- Same workflow for E. coli O157:H7, Listeria spp. & monocytogenes, Salmonella, Cronobacter,…
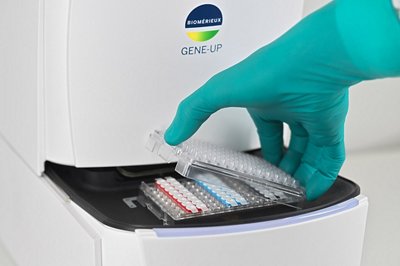
THE SIMPLEST INNOVATIVE WORKFLOW
The workflow of the GENE-UP® is very easy to use thanks to the patented Magic CAP® and the two pipetting step.

GENE-UP - THE SIMPLEST INNOVATIVE WORKFLOW
GENE-UP® FORMAT: WHEN SIMPLICITY BRINGS CONFIDENCE AND TRUST
GENE-UP® PCR FORMAT includes:
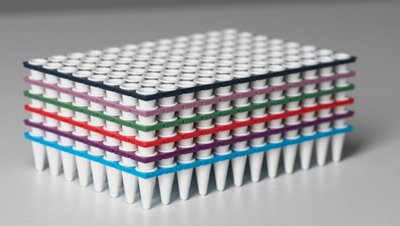
- Color coded PCR Plates. Increased visibility when handling multiple tests.
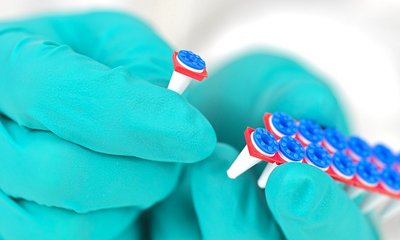
- PCR Septum caps, in combination with GENE-UP® DECAP-UP™. Increased quality of testing by securing lyophilized pellet in the well and reducing cross contamination risk.
- GENE-UP® PCR Tube Holder with Lid. Increased flexibility with breakable PCR plate and GENE-UP® PCR tube holder and lid: perform 1 to 96 tests at a time.


- New workflow
- Pcr color plates
GO BEYOND WITH CUSTOM MOLECULAR ASSAYS THANKS TO xPRO™ Program.
The xPRO™ Program is the catalyst that advances cutting-edge molecular diagnostics for quality and safety departments in the food, beverage, cannabis and dietary supplement industries. Industry leaders can partner directly with bioMérieux teams to rapidly develop, validate and commercialize molecular testing solutions to respond to emerging needs.
A PROVEN TECHNOLOGY
GENE-UP®, has three level of specificity with primers, FRET* probes and melt peak analysis.
The Mealt peak analysis offers an added level of interpretation valuable for sensitivity and accuracy.
(*) Fluorescence Resonance Energy Transfer
?qlt=85&ts=1716817692228&dpr=off)
FRET Schema
?qlt=85&ts=1716817578195&dpr=off)
3 Levels of Specifity
Tests
GENE-UP® solutions offer AOAC, Health Canada, AFNOR Certified* and MICROVAL validated test kits for your most pressing food safety concerns. The simple workflow is consistent for each test, minimizing training and improving efficiencies. Multiplex tests are available.
*AFNOR Certified – under the NF VALIDATION mark using the NF102 certification rules (available on the website https://nf-validation.afnor.org/en/).
Current Test Kits Include:
- Salmonella
- Duplex Salmonella Typhimurium & Salmonella Enteriditis
- Campylobacter spp
- E. coli O157:H7
- Cronobacter
- EHEC Solution
- STEC stx/eae
- STEC Top6
- PEC Pathogenic E.coli
- Listeria spp
- Listeria monocytogenes
- Norovirus GI + Norovirus GII
- Duplex Norovirus GI&GII
- Hepatitis A + Hepatitis E virus
- STEC - Salmonella
- Aspergillus
Services: Feasibility, Validation, and Instrument Support
Your productivity and product quality are our priority. At bioMérieux, we aren’t just selling fast and reliable solutions – our technical team and scientific experts are fully committed to help you get the most out of your equipment, to ensure the continuity of your operations.
From feasibility, to installation and IT integration and training, our entire range of services can be customized to your needs throughout your whole journey with us, focusing on your core business and optimizing your resources.
Downloads
GENE-UP® List of Validated Methods
Download
- Filename
- Validated-Methods-GENE-UP-Newsflash30.pdf
- Size
- 44 KB
- Format
- application/pdf
GENE-UP® Brochure
Download
- Filename
- BROCHURE_GENE_UP_PCR_PATHOGEN_DETECTION.pdf
- Size
- 5 MB
- Format
- application/pdf
- Filename
- GeneUp-MicroTally_EHEC_DataDigest_V3_Digital.pdf
- Size
- 124 KB
- Format
- application/pdf
- Filename
- GeneUp-MicroTally_EHEC_DataDigest_V3_Digital.pdf
- Size
- 124 KB
- Format
- application/pdf


















