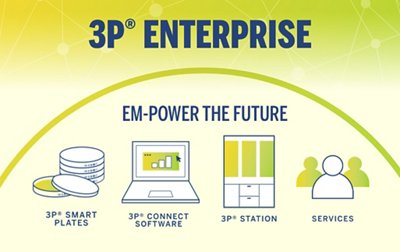
3P® ENTERPRISE
EM-POWER THE FUTURE
Bridging the gap between standard processes and automation, 3P® ENTERPRISE transforms the future of environmental monitoring in the pharmaceutical industry.
From sampling to trending, take control with an end-to-end, fully digitalized and automated workflow. Increase efficiency and ensure your products are safe and compliant with an innovative environmental monitoring system.



- Overview
- Technology
- Services
- Resources
Environmental Monitoring with 3P® ENTERPRISE
3P® ENTERPRISE is a fully integrated environmental monitoring (EM) solution designed to optimize your EM program and always keep your operations under control. The traceable culture media 3P® SMART PLATES are now enhanced with a GS1 barcode and clear design to enhance the reliability of your data. Tracking is continuous throughout the entire EM workflow through the user-friendly 3P® CONNECT SOFTWARE. Combined with the 3P® STATION, a system that automates the incubation and reading of standard Petri dishes, you reach end-to-end maximum efficiency and improve compliance.
Benefits
- IMPROVE PROCESS EFFICIENCIES: Time saving processes and faster responsiveness enable resources to be allocated more effectively to value-added tasks, and production capacity to be maximized.
- REDUCE ERRORS AND ASSOCIATED COSTS: User guidance at any time with alarms and conformity checks reduce the risk of errors & associated costs.
- BENEFIT FROM REAL TIME TRACEABILITY, SECURE DATA INTEGRITY AND COMPLIANCE: To be audit ready at any time with 21 CFR PART 11 compliance, ALCOA+ principle and cybersecurity by design.
- ENSURE ROUTINE USE AND CONTINUOUS OPERATIONS WITH MAXIMUM UPTIME: 3P® ENTERPRISE is a flexible, integrated solution that adapts to your organization. End-to-end services enable seamless, facilitated implementation and validation to ease and accelerate the uptime of the solution.
Bridging the gap between standard processes and automation, 3P® ENTERPRISE revolutionizes the future of EM. From sampling to trending, keep control with an end-to-end fully digitalized and automated workflow, increase your efficiency and ensure your products are safe and compliant.
MEET THE 3P® STATION.
To make the colony counting process less cumbersome and more robust, 3P® STATION has been developed with several high-level technical features specifically for pharmaceutical environmental monitoring:
- Robust reading performance: To ensure the best reading performance, the 3P® STATION uses a kinetic reading approach. Images of plates are taken every hour during the incubation cycle by a high-resolution camera. Your counting results are standardized and supervised in real-time, allowing you to be alerted as soon as a sample is out-of-specification.
- Enhanced samples traceability: Samples are tracked by their unique ID number. Data and images of the plates are available anywhere and anytime for review and archiving.
- Layout flexibility: Each 3P® STATION holds up to 300 plates and instruments can be flexibly laid out across QC lab or your manufacturing lines to fit with your process requirements and efficiency objectives.
- Incubation modes: The 3P® STATION has been developed to comply with your incubation practices whether you are using single temperature or double temperature cycles.
EASE OF USE
- Users are guided all along the workflow (scan plates, intuitive software and system)
- Supervision and validation of all samples facilitated
- Full paperless and automated EM process
ERROR AVOIDANCE
- Ensure expiration date, plate type, sampling & incubation durations to avoid errors
- Benefit from a real-time, complete traceability and tracking of samples
- Rely on alerts and alarms along the process
DATA ANALYSIS & REPORTING
- Full and centralized data
- Trending and reporting
- Audit trail & alarm reports
- Production and laboratory view for supervision of samples
Support And Services Where And When You Need
Your productivity and product quality are our priority. At bioMérieux, we aren’t just selling fast and reliable solutions – our technical team and scientific experts are fully committed to help you get the most out of your equipment, to ensure the continuity of your operations.
Our dedicated teams are here to support you throughout your transformation. As your partner, we’re committed to providing you with the assistance and services you need at every step of your operations journey, from implementation to routine use.
BROCHURE - Creating Value by Harvesting the Full Potential of your Bioprocessing Testing
DISCOVER OUR COMPREHENSIVE PORTFOLIO
- Filename
- BMX_Bioproduction_Brochure_A4_vF_web.pdf
- Size
- 7 MB
- Format
- application/pdf
BROCHURE - Our Comprehensive Portfolio
DOWNLOAD BROCHURE
- Filename
- BMX_Bioproduction_Brochure_A4_vF_web.pdf
- Size
- 7 MB
- Format
- application/pdf
BROCHURE - OPTIMAL - Service Contract
DOWNLOAD BROCHURE
- Filename
- BMX_Partnership_Assist_Brochure_A4_v6_final_DIGITAL (1).pdf
- Size
- 1 MB
- Format
- application/pdf
BROCHURE - Cybersecurity
DOWNLOAD BROCHURE
- Filename
- bioMerieux_3PEnterprise_CyberSecurity_Brochure.pdf
- Size
- 1 MB
- Format
- application/pdf
Explore All 3P® Solutions
You may also like...
Insights
- Teaser 1
- Teaser 2
- Teaser 3
- Teaser 4