Embrace the Future of Microbial Detection in Cell and Gene Therapy with USP <72>
Automated growth-based methods have long been utilized to test microbial contamination across a diverse range of pharmaceutical products, including cell and gene therapy (C>) products. These innovative therapies are revolutionizing the treatment of life-threatening and chronic diseases, offering significant benefits to patients. Due to their short shelf life, these products must be administered promptly and tested for microbial contamination to ensure patient safety.
Recognizing the need for rapid microbiological methods, the United States Pharmacopeia (USP) has published a new chapter, USP <72>, which focuses on respiration-based microbiological methods for detecting contamination in short-life products. This chapter represents a significant advancement in championing rapid methods, which are widely recognized for their reliability and ability to deliver timely, actionable results.
Advantages of Respiration-Based Methods
Respiration-based microbiological methods, as described in USP <72>, offer several advantages over both traditional sterility testing methods outlined in USP <71>, Ph. Eur. 2.6.1, JP 4.06, and ATP-based methods referenced in USP <73>:
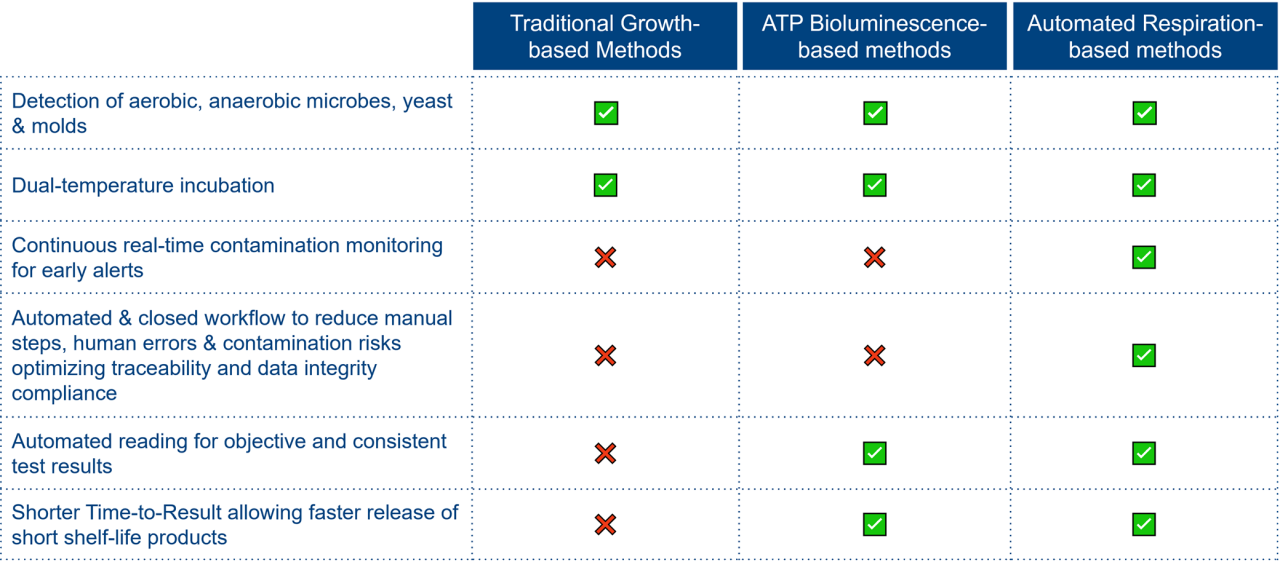
Faster product release through flexibility
Accelerating product release starts with optimizing time to detection — and USP <72> enables it. This chapter offers manufacturers the flexibility to tailor culture media and incubation conditions based on risk and process parameters. By leveraging this flexibility, you can significantly shorten your time to detection.
At bioMérieux, we help you make the most of this opportunity. Our recent data (in the absence of product interference) show that with optimized incubation, a respiration-based method like BACT/ALERT® 3D can detect contamination in as little as 4 days. View our poster here.
A proven technology with streamlined validation
The fact that all commercialized CAR-T therapies are currently released using the BACT/ALERT® system is a clear indication of trust in its reliability and performance. Including BACT/ALERT® as a reference method in USP <72> opens the door for its use across a broader range of therapies that require rapid, reliable microbial testing by simplifying validation requirements.
Conclusion
USP <72> will become official on August 1, 2025, marking a significant milestone in the advancement of microbial detection for short-life products. This chapter empowers manufacturers to significantly reduce time-to-release, enabling faster delivery of critical therapies to patients in need. Automated methods such as BACT/ALERT® support this by providing faster, more efficient, and reliable microbial testing.
Join us in embracing the future of microbial detection in cell and gene therapy with USP <72>!
Interested In Automated Rapid Sterility Testing with BACT/ALERT® 3D?
Complete the form below to get in touch with our team. A bioMérieux expert will be in touch using the contact information you provide. By providing your information, you agree to receive additional emails from bioMérieux about sterility testing.
To find out more about the management of your personal data and to exercise your rights, see our privacy policy.


