Bacterial Endotoxin Testing: 10 Reasons to Choose Recombinant Factor C
Recombinant horseshoe crab Factor C (rFC) methods are the latest state-of-the-art solution for effective bacterial endotoxin testing (BET). This whitepaper reviews the advantages of Recombinant horseshoe crab Factor C (rFC) over the BET methods currently in widespread use. We compare the performance of LAL reagents with rFC, and summarize the evidence supporting our 10 reasons to choose rFC.
BACKGROUND
Macrophages, a type of immune cell, use the lipopolysaccharides (LPS) molecules that make up the outer cell wall of gram-negative bacteria to detect their presence in bodies. LPS, also known as bacterial endotoxins, can elicit a severe response in the immune system, resulting in fever, hypotension, nausea, shock, and sepsis.
Severe reactions to bacterial endotoxins can be fatal, and as such, great care must be taken to ensure that they don’t find their way into medical products making contact with a patient’s bloodstream or cerebral fluid1,2. Unfortunately, avoiding endotoxin contamination during pharmaceutical manufacturing processes is by no means an easy task, because LPS molecules are present in virtually every environment. As a result, there are strict regulations for the acceptable levels of endotoxin contamination in medical devices, injectable pharmaceuticals, and other medical
solutions that may come into contact with a patient’s blood or cerebral fluid. Such products must, therefore, be tested for endotoxin contamination before they can be released3.
A BRIEF HISTORY OF ENDOTOXIN TESTING WITH LAL
In the 1960s, scientists discovered that the isolated lysate from the Atlantic horseshoe crab (Limulus Amebocyte Lysate -LAL) coagulated when in the presence of bacterial endotoxins. Around 10 years later, researchers also found that the same process occured in the isolated lysate from the Asian horseshoe crab (Tachypleus Amebocyte Lysate - TAL). Bouyed by their discovery, they devised tests aimed at detecting bacterial endotoxins using LAL and TAL reagents, which were then adopted by the US Pharmacopeia in 19832,4.
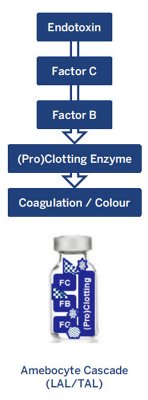
Three approved main test methodologies utilize LAL and TAL reagents for endotoxin testing: Gel Clot (limit and semi-quantitative), Turbidimetric, and Chromogenic (end-point and kinetic methods) assays. The LAL/TAL tests all work in a similar fashion; the presence of bacterial endotoxins in a sample sets off a cascade of reactions, resulting in a change of turbidity or color (see Figure 1)4,5.
The amebocyte lysate contains a mixture of naturally occurring proteins that are involved in the endotoxin detection process. Factor C acts as the principal biosensor by binding with bacterial endotoxins, activating another protein called Factor B. Factor B then converts a pro-clotting enzyme to a clotting enzyme.
The resulting clotting enzyme then catalyzes a reaction that causes a change in viscosity, turbidity, or
color, which is detected to determine the concentration of endotoxins in the sample4,5.
LAL: ENVIRONMENTAL IMPACTS AND LIMITATIONS
Although LAL and TAL are widely used in the pharmaceutical industry (principally because they provide sensitive detection of endotoxins), they have several disadvantages. LAL and TAL tests are susceptible to false-positives, and furthermore, the relatively high batch-to-batch variation found in natural lysate reagents reduces their reliability and comparability4,6.
Perhaps the most significant problem with LAL and TAL reagents is that they must be obtained from horseshoe crabs. The Atlantic horseshoe crab population has declined by 90% over the last 15 years, and the species is now described as vulnerable by the IUCN Redlist of threatened species. The Asian horseshoe crab population has been similarly affected, and is now considered endangered6,7.
As pharmaceutical sales and manufacture continue to grow, so too does the demand for endotoxin testing reagents. Declining numbers of horseshoe crabs mean using LAL and TAL to test for bacterial endotoxins no longer sustainable. Furthermore, LAL and TAL are only produced in certain regions, and their availability is, as such, limited in some areas across the globe6.
To ensure the continued safety of our pharmaceuticals, it is clear that we need an alternative endotoxin test that is not only sustainable, but also widely available, and able to meet increasing endotoxin testing demands.
DOWNLOAD THE WHITE PAPER TO READ THE FULL ARTICLE
Complete the form below to access the white paper, which will be sent directly to your email after submission. By providing your information, you agree to receive additional emails from bioMérieux about endotoxin testing.
To find out more about the management of your personal data and to exercise your rights, see our privacy policy.


?qlt=85&ts=1749133251187&dpr=off)
