Data Integrity Management and Risk Mitigation Made Simple
⏱Read time: 3 minutes
The standards for proving data integrity have gotten stricter. Today, regulatory organizations from all over the world, such as the FDA, EMA, and PDMA, conduct audits more frequently, in a more extensive way, and observations are becoming more frequent. They demand that data is accurate and reliable. It’s in the best interest of pharmaceutical companies to design flexible and risk-based strategies to ensure data integrity.
Robotic applications have the potential to analyze and improve business processes based on reliable data. It supports faster time-to-market and helps increase production efficiency.
When it comes to endotoxin testing, the same standards for high throughput, high quality, and high integrity testing are expected.
In keeping with the desire to meet 3R parameters (reduce, reuse, replace the need for animal-derived ingredients), a new solution highlighted by bioMérieux provides advantages that reduce user hands-on time and cost per test, employ sustainable rFC reagents, and thus eliminates the need to harvest horseshoe crabs for their blood or to increase plastic usage as with microfluidic cartridge-based endotoxin testing technologies.
The ability to process one hundred and twenty (120) samples of endotoxin tests in an hour is a huge advancement and unparalleled in the industry to date. This offers a powerful, high-throughput solution for the endotoxin market.
The Tecan Fluent® workstation has been paired with the bioMérieux ENDOZYME® II GO pre-coated plates to create a seamless automation solution for endotoxin testing. The basic workflow can be seen below.
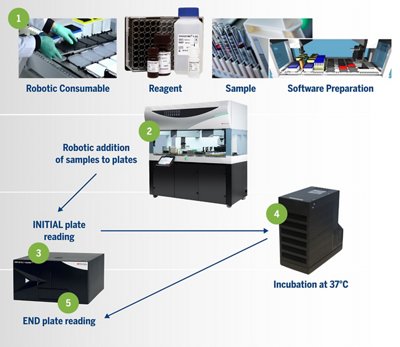
- Robotic consumable/reagent/sample/ software preparation
- Robotic addition of samples to plates
- INITIAL plate reading
- Incubation at 37°C
- END plate reading
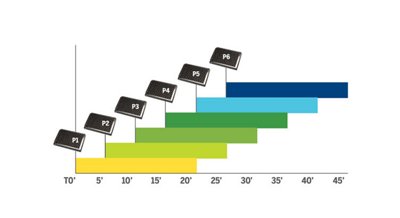
6 plates can be processed in parallel
100 to 120 samples in less than 1 hour*
*For a 0.05 EU/mL sensitivity; 100 to 120 samples for a 0.005 EU/mL sensitivity
The test takes advantage of the endpoint fluorescent basis of the rFC test. After the initial read, while the plate is not being read, the robot places the plate into an attached incubator and proceeds with the next initial plate read. This is repeated for up to 6 tests in one hour.
An additional advantage of the ENDOZYME® liquid rFC reagent is that it is extremely stable at room temperature, meaning it can sit out for at least 8 hours in a transparent tube, while other recombinants and LAL products have approximately only 30 minutes of stability at room temperature.
Data Integrity/Error Reduction
The new robotic offering employs the use of ENDOZYME® II GO plates that provide pre-spiked product control wells (PPCs) and pre-loaded standard curve wells which can be reconstituted by simply adding endotoxin-free water. In this way, plates are used that already have the expectation of standard curve and PPCs that are always in range. This method, automation within automation, streamlines user-performed activities to the bare minimum. Minimizing manual input is a key step in enhancing workflow reliability, reducing the chances of human error, and ensuring smoother performance. Optimizing automation is straightforward—simply add your samples."
The complete solution includes the following capabilities:
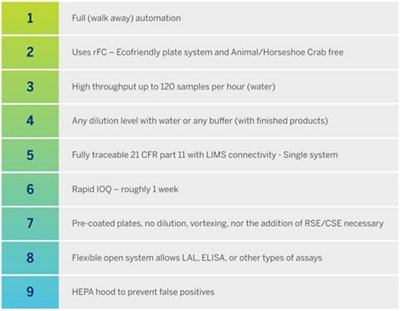
Organizational improvements apply to both, (i) the physical organization and manipulation of consumables, reagents, and samples and to (ii) the data and meta-data gathering and capabilities. These organizational improvements largely fulfill the ALCOA expectations built around CFR Part 11 compliance expectations.
ALCOA is an acronym for Accurate, Legible, contemporaneously created, Original, and Attributable electronic records for review and approval.
In this way, it is clearly superior in excluding the possibility of injecting human actions. From the scheme in Figure 1, the entire process becomes an interface for human interaction in a more organized and efficient way and in a manner that facilitates regulatory compliance.
The MOST Expensive Test...
It is often said that the most expensive test is a test that must be repeated. Reducing the error rate by reducing handling errors is only one of the advantages of using a pre-spiked plate:
- No reconstitution of CSE vial, dilution, and mixing (reduce false positives due to lab exposure to CSE aerosol)
- The standard curve is already pre-coated on each plate (no manually prepared serial dilution necessary)
- No addition of tiny 10 mL sample spikes (already added to wells)
- Concentrates the analyst’s attention to properly preparing the samples for the test and completing necessary documentation steps