A Rapid, Fully Automated Mycoplasma Detection System
Authors:
WILLIAM E. BARRY 1, CORIKE TOXOPEUS 1, JESSICA BROWN 1, CYNTHIA D. ANDJELIC 1,
SYLVANIE CASSARD 2, FÉLIX A MONTERO JULIAN 2, MARIANNE KIM 1, CYNTHIA L. PHILLIPS 1
Routine testing for microbial contaminants is a necessary part of ensuring biopharmaceutical product quality and safety. In this white paper, we describe the potential application of BIOFIRE FILMARRAY® – a well-recognized technology used in infectious disease diagnostics for rapid at-line detection of Mycoplasmas in the pharmaceutical industry.1
Mycoplasmas (i.e. Mollicutes) are notorious microbial contaminants of eukaryotic cell cultures that are particularly difficult to detect and eliminate. Their presence can negatively impact the health of cells in culture, decrease bioreactor yields, interfere with in vitro tests, and in some cases cause disease.2,3 Compendial Mycoplasma test methods rely on bacterial culture techniques, which take up to 28 days to obtain results.
Not only do these methods require substantial incubation time, but propagating live Mycoplasma cells also presents significant contamination risk to the manufacturing facility that may necessitate offsite or outsourced testing. Accordingly, Mycoplasma testing represents a common bottleneck in the manufacturing process, and in the case of short shelf-life products such as cellular therapies, compendial Mycoplasma tests may not be suitable.
Recognizing the need for more rapid test methods, pharmacopeias have provided guidance on the use of Mycoplasma Nucleic Acid-based Tests (NATs) as an alternative to compendial methods for lot release testing.4-6 With results potentially available within days or hours, rather than several weeks, the use of Mycoplasma NATs also present a possibility for in-process testing as part of broader contamination control strategies to reduce risk and improve decision making throughout the manufacturing process (consistent with PAT and QbD initiatives). However, conventional NATs still require a significant amount of hands-on time from highly skilled operators, along with a dedicated laboratory, such that testing at the manufacturing line has not yet been feasible for near real-time Mycoplasma contamination monitoring.
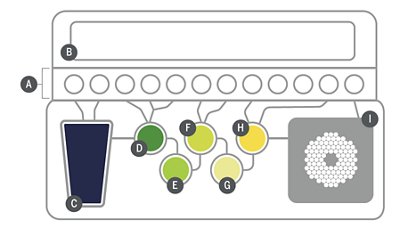
In contrast to conventional NATs, the BIOFIRE® Mycoplasma Solution (Figure 1) provides a closed and fully-automated ‘lab in a pouch’ NAT system that requires approximately five minutes of hands-on time with minimal user training and skill. The BIOFIRE Mycoplasma Pouch (Figure 2) is a closed disposable cartridge that stores all the necessary reagents for automated cell lysis, nucleicacid purification, reverse transcription, first and second stage polymerase chain reactions (PCR), and analyte detection in order to isolate, amplify, and detect Mycoplasma nucleic acid from a single sample.

Figure 1
The pouch runs on the compact FILMARRAY® 2.0 instrument, which automates all steps from cell lysis through analyte detection, with final results available in approximately 45 minutes after initiating the run. Pouch and sample information can be scanned to the instrument by the operator using a hand-held bar code reader, or entered manually. At the end of each run, an automated test report is produced to inform the user whether or not Mycoplasma was detected in the sample.
Each pouch includes fourteen different PCR assays to cover the wide genetic diversity of the Mollicutes class, with more than 130 species detected. Three internal controls ensure the reliability of results by monitoring all aspects of pouch function, from sample extraction through amplicon detection. The system was designed to meet compendial testing standards, with the Limit of Detection (LoD) studies performed for Mycoplasmas listed in the United States, European, and Japanese pharmacopeias using 10 mL and 0.2 mL sample volumes. The LoD values for 10 mL samples range from <1 CFU/ mL to 10 CFU/mL for all ten compendial Mycoplasmas tested, with only a few simple pre-processing steps by centrifugation required. Direct testing of 0.2 mL samples provides LoD values ranging from 1 to 33 CFU/mL and is intended to facilitate more frequent surveillance of raw materials, cell banks, early product intermediates, and biologics with limited volume availability.
Specificity was evaluated by testing high concentrations of non-target bacteria closely related to the Mollicutes class, including species of Bacillus, Clostridium, Lactobacillus, and Streptococcus. No false positive results were observed at ≤1E+06 CFU/mL for all organisms tested, demonstrating high specificity of the test. In addition, a range of different substances related to biopharmaceutical manufacturing were tested for possible interference with result accuracy.
To this end, substances were tested both unspiked (blank) and spiked with five different Mollicutes (A. laidlawii, M. fermentans, M. orale, M. pneumonia, S. citri) at 3xLoD. No interference with internal controls was observed for any test substance and all Mycoplasmas were successfully detected, with the exception of bleach, which was found to interfere with Mycoplasma detection when introduced into the sample at 1% of the final sample volume. Extra care should therefore be taken in the case that bleach is used near the site of testing or sample collection.
Robustness of the BIOFIRE® Mycoplasma Solution was evaluated by making small variations in method parameters that may be introduced by operators during normal use, which showed no erroneous Mycoplasma test results. Ruggedness testing also confirmed the lack of influence from operational and environmental variables on th reproducibility of test results, including different analysts, different pouch lots, different FILMARRAY® Instruments, and multiple test days.
Taken together, the highly sensitive and specific analytical performance, rapid automated results, and compact system design of the BIOFIRE Mycoplasma Solution presents a unique potential for at-line in-process Mycoplasma testing with near real-time results. With approximately five minutes of hands-on time and automated result reporting in approximately one hour, the system simplifies the implementation of in-house Mycoplasma testing to eliminate a common manufacturing bottleneck and improve Mycoplasma surveillance throughout the manufacturing process.
Key Features of the BIOFIRE® Mycoplasma System
Easy: Less than five minutes of hands-on time; one test panel per sample.
Fast: Instrument run time and test results available in under 1 hour.
Broad Detection: Covers genetic diversity across the Mollicutes class.
Sensitive: Detection of compendial Mycoplasma at ≤10 CFU/mL.
Closed System: Automated sample extraction, nested multiplex PCR, results reporting, and reduced contamination risk.
Sample Volume Flexibility: Simple sample pre-processing allows sample volumes ranging from 0.2 mL to 10 mL, or even higher.
Room Temperature Stable: Store reagents at 18-30°C
Compact Instrument: 25.4 x 39.3 x 16.5 cm (10 x 15.5 x 6.5 in)
Software: 21 CFR 11 compliant
References
- Poritz, M.A., Anne J. Blaschke, A.J., Byington, C.L., Meyers, L., Nilsson, K., Jones, D.E., Thatcher, S.A., Robbins, T., Lingenfelter, B., Amiott, E., Herbener, A., Daly, J., Dobrowolski, S.F., Teng, D.H.F., Ririe, K.M., (2011) FILMARRAY, an Automated Nested Multiplex PCR System for Multi-Pathogen Detection: Development and Application to Respiratory Tract Infection, PloS ONE Vol (6)10: 1-14.
- Drexler HG, Uphoff CC. Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. Cytotechnology. 2002;39(2):75-90. doi:10.1023/A:1022913015916.
- Armstrong SE, Mariano JA, Lundin DJ. The scope of Mycoplasma contamination within the biopharmaceutical industry. Biol J Int Assoc Biol Stand. 2010;38(2):211-213. doi:10.1016/j.biologicals.2010.03.002.
- US Pharmacopeia <63> Mycoplasma Tests. In: USP 39 - NF 34. United States Pharmacopeial Convention; 2016:130-136.
- 2.6.7. Mycoplasma. In: European Pharmacopoeia. 9.0. The European Directorate for the Quality of Medicines & HealthCare; 2016:188-193.
- Mycoplasma Testing for Cell Substrates used for the Production of Biotechnological/Biological Products. In: The Japanese Pharmacopoeia. 17th ed. Pharmaceuticals and Medical Devices Agency; 2016:2460-2464.

