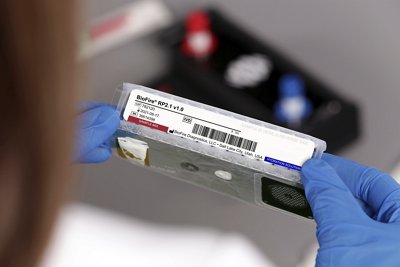
The BIOFIRE® Respiratory 2.1-EZ (RP2.1- EZ) Panel (EUA)*
The BIOFIRE RP2.1-EZ Panel (EUA)* uses a syndromic approach to quickly identify SARS-CoV-2, along with 18 additional viral and bacterial pathogens with results in about 45 minutes.
Product not available outside of the U.S.
- Product Overview
- Technical Details
- Service & Support
- Resources
Product Overview
1 Test. 19 Targets. ~45 Minutes.
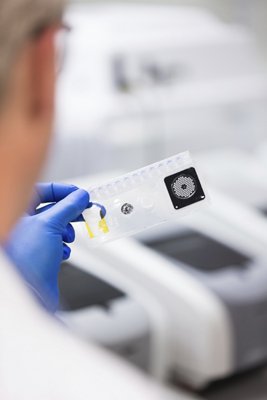
Now authorized by the FDA through an Emergency Use Authorization, the BIOFIRE RP2.1-EZ Panel (EUA)* uses a syndromic approach to quickly identify SARS-CoV-2, along with 18 additional viral and bacterial pathogens in patients suspected of SARS-CoV-2. This PCR test provides results in about 45 minutes. As a healthcare provider, this means your patients can receive the right treatment the first time, which may lead to shorter appointment times and improved antimicrobial stewardship. The BIOFIRE RP2.1-EZ Panel (EUA)* is designed to run on the BIOFIRE® FILMARRAY® 2.0 EZ Configuration System, which is intended to be used in outpatient settings.
Onsite Respiratory Diagnostics
Traditionally, clinical care of respiratory tract infections is designed around syndromic disease management and centralized laboratory testing.1 Now laboratory testing is shifting to decentralized settings, creating a simplified process with faster results.2 With the shift toward value-based care, syndromic testing from bioMérieux can help you do what is best for the patient while reducing untimely and expensive send-out tests. The BIOFIRE RP2.1-EZ Panel (EUA)* may help improve clinician confidence in diagnosis, aid in antimicrobial stewardship, improve patient management, enhance diagnostic yield, and reduce appointment duration.

Appropriate Treatment Relies on Knowing, Not Guessing
Among ambulatory care visits in the U.S., only 50% of antibiotic prescriptions for acute respiratory infections were estimated to be appropriate.3 In 2016, to combat the growing threat of antibiotic resistance, the President’s National Action Plan called for a goal of reducing antibiotics prescribed for acute respiratory conditions by 50% in 2020.4
Rapid and comprehensive test results from the BIOFIRE RP2.1-EZ Panel (EUA)* may help you discuss the efficacy of antibiotics with your patients. Timely diagnosis may increase patient satisfaction while potentially reducing unnecessary antibiotic prescriptions.
Furthermore, when patients have clear answers about what’s causing their respiratory infection, they may be less likely to seek additional care in the emergency department.
Don’t Guess. Know.
At bioMérieux, we take a syndromic approach to infectious disease testing. The BIOFIRE RP2.1-EZ Panel (EUA)* uses multiplex PCR technology to quickly and accurately identify a comprehensive menu of respiratory pathogens that can cause similar symptoms in patients suspected of SARS-CoV-2. Molecular testing is more sensitive than culture and more efficient than individual and send-out tests.

Technical Details
- Sample Type: Nasopharyngeal swab in transport media or saline
- Overall 97.1% sensitivity | 99.3% specificity (prospective specimens)5
- SARS-CoV-2 98.4% PPA and 98.9% NPA6
Panel Menu:
VIRUSES:
- Adenovirus
- Coronavirus 229E
- Coronavirus HKU1
- Coronavirus NL63
- Coronavirus OC43
- Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
- Human metapneumovirus
- Human rhinovirus/enterovirus
- Influenza A virus
- Influenza A virus A/H1
- Influenza A virus A/H3
- Influenza A virus A/H1-2009
- Influenza B virus
- Parainfluenza virus
- Respiratory syncytial virus
BACTERIA:
- Bordetella parapertussis
- Bordetella pertussis
- Chlamydia pneumoniae
- Mycoplasma pneumoniae
Service & Support
With the urgent nature of patient and community care, we take pride in addressing all concerns quickly and accurately. bioMérieux is dedicated to providing world class customer support 24 hours a day, 7 days a week, 365 days a year.
For assistance please contact our customer technical support team at:
- Email: biofiresupport@biomerieux.com
- Phone: 801-736-6354 option 5
- Toll Free: 1-800-735-6544
To order, contact your regional sales representative or use the following contact information:
- Phone: +1-801-736-6354 x 1502
- Fax: +1-801-588-0507
- Email: CSCFax@biomerieux.com
- International Sales: +1-801-736-6354 x 1536
- Military Sales: 1-801-262-3592
Resources
Technical & Supporting Documents
Videos
How the BIOFIRE® Respiratory Panels transform care for pediatric patients
BIOFIRE Torch System
BIOFIRE Torch System
References:
- Schreckenberger PC, McAdam AJ. J Clin Microbiol.2015;53:3110-3115.
- Jani IV et al. NEJM2013;368(24):2319-2324.
- Fleming-Dutra KE et al. JAMA.2016;315(17):1864-1873
- Pew Charitable Trust Report. Antibiotic Use in Outpatient Settings. 2016
- Based on the archived specimen study in the BIOFIRE Respiratory 2.1 (RP2.1) Panel EUA submission.
- Based on the contrived specimen study in the BIOFIRE Respiratory 2.1 (RP2.1) Panel EUA submission.
*This product has not been FDA cleared or approved, but has been authorized for emergency use by FDA under an EUA for use by authorized laboratories; This product has been authorized only for the detection and differentiation of nucleic acid of SARS-CoV-2 from multiple respiratory viral and bacterial organisms; and, The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug, and Cosmetic