How to Control the Risk of Cross-contamination in Food Microbiology Laboratories?
BIOBALL® LUMINATE 2.0 brings precision, accuracy and innovation to food industrials to ensure microorganism quality control in their products.

Bacteria, along with fungi and virus, are the top 1 enemy of food industrials. When they contaminate food products, consequences can be catastrophic for both consumers and industrials. Even if regulations are increasingly strict, food safety scandals regularly break the news, thus reducing the confidence of consumers in food products found on supermarket shelves.
Before the supermarket, in the production sites, contaminations usually occur from raw foods or environment to ready-to-eat products that do not undergo further processing to eliminate bacteria. Therefore, microbiological contamination has been a contributing factor to several outbreaks of foodborne illness, and to countless product recalls.
Contamination in a food facility can come from various sources, including the absence of strict hygiene conditions and the lack of monitoring of quality indicators. More unusually, a contamination due to bacteria used in the laboratories of the food industrial itself can be detected.
Indeed, food industrials frequently use collection strains, mainly for verifying and validating their methods (ISO 16140-3 and ISO 16140-4) as well as preparing positive tests for process controls to guarantee the reliability of their results.
When preparing these positive tests for process control or to verify and validate methods, bacteria from culture collections can contaminate the tested samples during testing protocol, leading to false positive results. It is what we call a cross-contamination, defined as the unintentional transfer of microorganisms from one item (e.g. a collection strain) to another one (e.g. a tested sample).
Even if these bacteria used in microbiological laboratories can hardly be found in finished products of the production area, they can create false positives. Positive results trigger unnecessary alerts in production sites which results in an additional workload, a loss of time and therefore of money, especially when they are not due to a natural contamination of the products.
It comes as no surprise that the pathogenic bacteria most likely to create problematic false positives with huge impact are those routinely used by food industrials for their detection methods of quality control. Among these bacteria, five strains have been identified as the most challenging for food industrials.
The Most Problematic Bacteria Isolated in Food Laboratories
1. Listeria monocytogenes
Listeria is a genus of Gram-positive bacteria, belonging to the Listeriaceae family and composed of 27 species, including Listeria monocytogenes. This species is the only one to be pathogenic for humans as it is responsible for one of the most severe foodborne diseases: listeriosis.
2. Listeria innocua
Listeria innocua is a non-pathogenic strain that behaves similarly to L. monocytogenes as they belong to the same sensu stricto group and are found in similar environments. The main phenotypic characteristic that distinguishes it from L. monocytogenes is that it is not hemolytic, making L. innocua the most frequently encountered non-pathogenic Listeria species in food production environment.
3. Cronobacter sakazakii
Cronobacter is a genus of Gram-negative opportunistic pathogens, composed of 7 species, including Cronobacter sakazakii. This species causes meningitidis, septicemia and necrotizing enterocolitic in neonates and infants, with neurological sequelae in severe cases. Interest in Cronobacter has remained significant due to its high virulence in children, especially through the consumption of contaminated infant formulas.
4. Salmonella Typhimurium
Salmonella is a Gram-negative rods genus belonging to the Enterobacteriaceae family. Within two species, Salmonella bongori and Salmonella enterica, over 2500 different serotypes have been identified, including Salmonella enterica serotype Enteritidis and serotype Typhimurium. These two serotypes are the main cause for salmonellosis, a disease that can become severe and life-threatening for vulnerable people.
5. Escherichia coli O157:H7
Escherichia coli O157:H7 is a serotype of the well-known Escherichia coli species and more specifically one of the Shiga-like toxin producing Escherichia Coli (STEC). These specific bacteria are major foodborne pathogens responsible for human illnesses, characterized by non-bloody to bloody diarrhea, sometimes leading to complications, particularly in children. Escherichia coli O157:H7 is even the major serotype responsible for many of the STEC illness outbreaks in humans.
How BIOBALL® LUMINATE 2.0 Limits the Risk of False Positives in Food Laboratories
As evidenced by the long list of clinical signs caused by these bacteria, contaminations of food products can be a serious risk for public health. Therefore, positive samples, especially positive food samples, are always taken seriously and trigger a series of actions.
By using sensitive and specific methods to detect and enumerate these pathogens, food industrials verify that no pathogens present in the tested samples are missed.
By using BIOBALL® LUMINATE 2.0 for control process applications, food industrials go one step further by giving themselves the possibility to verify that no false positives are released due to cross-contamination.
Use of Precise and Dedicated Strains for Food Applications
In order to reduce this risk of false positives, food industrials have now the possibility to use easily distinguishable strains, such as fluorescent bacteria, as authorized in the future revision of ISO/DIS 7218.
To help industrials to follow this recommendation, bioMérieux has developed in his state-of-the-art laboratories in Australia a range of genetically altered strains specifically designed for quality control, validation and verification of methods in the food field. To answer the most common needs of the food industry, this range exists for the five strains described above.
BIOBALL® LUMINATE 2.0 are small, water-soluble balls, ideally designed for positive controls and verification of methods according to ISO 16140-3. They are also easy to use as they require no time-consuming preparation or culture, thus limiting the risk of cross-contamination.
Unlike other products on the market, each BIOBALL® LUMINATE 2.0 contains a highly precise count of 100 cfu with a SD of 15% of the mean. Therefore, they can be used straight from the freezer or after a simple dilution, making them the easiest to use on the market.
In order to always offer reliable products, BIOBALL® LUMINATE 2.0 is a Certified Reference Material (according to ISO 17034), as well as the standard BIOBALL®.
Use of the Green Fluorescent Protein to Highlight False Positives
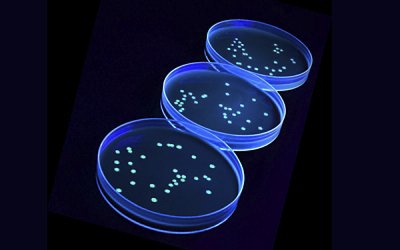
The Green Fluorescent Protein (GFP) is what makes these new BIOBALL® LUMINATE 2.0 special. Each of them is tagged with a GFP directly integrated into the chromosome of the five host strains, making them highly stable and easy to distinguish from natural contaminants.
To observe this genetic marking, BIOBALL® LUMINATE 2.0 works with two confirmation techniques, including the use of UV light to observe their fluorescence after culture and INVISIBLE SENTINEL® kit running on VERIFLOW® platform to detect specific GFP insertions.
By using BIOBALL® LUMINATE 2.0, food industrials can confirm with one of these confirmation techniques that positive samples are not due to cross-contamination from the laboratory, but are true positives needing immediate actions. However, if a cross-contamination is confirmed, further testing is required to confirm that the sample does not contain any targets.
BIOBALL® LUMINATE 2.0 as a Key Solution for Food Industry Microorganism Quality Control
BIOBALL® LUMINATE 2.0 GFP strains are now the best allies of food industrials to limit the risk of releasing false positives and to advantageously replace collection of these pathogenic microorganisms from the laboratory.
BIOBALL® LUMINATE 2.0 is a range of Genetically Modified Microorganisms (GMM) and may need to comply with special regulatory requirements for a laboratory contained use in your country (e.g., European directive 2009/41/EC completed by national regulation). In this case, please, refer to your local competent authority to declare or obtain an agreement before use.
Discover Also
- Discover Also