Business development partner
The Companion Diagnostics program partners with pharmaceutical, vaccine and medical device companies regarding their clinical therapy development for better patient outcomes.
We partner with pharmaceutical, vaccine and medical device companies regarding their clinical therapy development for better patient outcomes.
The value of diagnostics to meet pharma challenges and expectations
Pharmaceutical companies challenges & expectations
Value of diagnostics
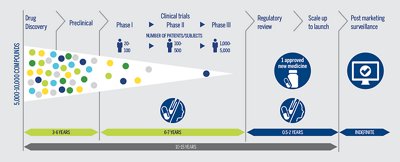
CLINICAL TRIALS
Clinical trials are long, costly, and could fail. Maximize the probability of trial success while optimizing time and cost.
Optimize clinical trials by selecting the appropriate patient population leading to an increased probability of trial success, as well as timesavings and cost-effectiveness by limiting unnecessary patient drug exposure.
COMMERCIALIZATION
Personalized Medicine is crucial for innovative drugs : “The right treatment for the right patient at the right moment and with the right dose.”
Ensure the most appropriate choice of drug in a given context to improve the chances of positive patient outcome while avoiding/ limiting undesirable side effects, leading to safe drug adoption and prescription.
Personalized medicine focuses on giving the right treatment to the right group of patients at the right time, based on an in-depth understanding of the patient’s medical status and specific biological criteria.
Supported by companion and supportive/complementary diagnostics, its aim is to improve the chances of a positive patient outcome by administering targeted interventions only to those patients who are likely to respond (i.e. those for whom the treatment will most likely be effective), while avoiding or limiting undesirable side effects as well as optimizing the cost-efficacy ratio.
Personalized medicine is already being widely used in oncology. In the future, it is poised to undergo significant development in other therapeutic areas: infectious diseases, neurology, immunology, cardiology, gastroenterology, etc.
Meet us at ECCMID 2024!
✉️ Contact our teams at: companion.diagnostics@biomerieux.com
📖 Download our Companion / Supportive Diagnostics brochure


