bioMérieux Named Business of the Year by Utah Governor’s Office of Economic Opportunity
bioMérieux, a world leader in the field of in vitro diagnostics, has received the 2024 Business of the Year Award, presented by the Utah Governor’s Office of Economic Opportunity at One Utah Summit April 11-12, in Salt Lake City.
Each year, at the One Utah Summit, Utah’s Governor recognizes outstanding individuals and companies for their significant contributions to Utah’s economy, local community, and industry. This year, bioMérieux was chosen for the Business of the Year Award, recognizing the company’s contributions and innovations in Utah.
“Our teams work hard every day to help make the world a healthier place. This award spotlights the innovative and pioneering spirit of our team members in the region and across the world,” said Colin Hill, General Manager, bioMérieux North America. “We proudly accept this award on behalf of our committed team members. We are honored to have been chosen as Business of the Year among so many successful companies in Utah.”

Colin Hill, General Manager, bioMérieux North America receiving the 2024 Business of the Year Award
About bioMérieux’s presence in Utah
bioMérieux established a presence in Salt Lake City in 2014, with its acquisition of BioFire Diagnostics, an innovative company that had pioneered an easy-to-use, sample-to-answer, and comprehensive molecular approach to infectious disease diagnostics. Salt Lake City is now home to bioMérieux’s North America Headquarters. According to BioUtah, we are the second largest life sciences employer in the state, with 3,500 team members spread across six sites.
Since 2014, bioMérieux has invested more than $200 million in its Salt Lake City operation, which includes six sites. Major investments include:
- Global Center for Molecular Diagnostics in Research Park
- North America Headquarters, located south of the international airport
- State-of-the-art manufacturing center, now employing 1,300 team members and operating 24/7

Salt Lake City, Utah
The place of birth of our latest advancement in molecular infectious disease diagnostics
Bringing diagnostic results as close to patients as possible. Cleared for the market by the US FDA in 2023, the BIOFIRE® SPOTFIRE® solution allows care for patients suspected of respiratory tract infections with comprehensive results delivered during a patient’s visit in approximately 15 minutes.
Three tests for use on BIOFIRE® SPOTFIRE® have already received (510)k accreditation from the U.S. FDA, as well as a CLIA-waiver: Respiratory (R) Panel, Respiratory (R) Mini Panel and Respiratory/Sore Throat (R/ST) Panel.
From ideation through manufacturing, the BIOFIRE® SPOTFIRE® system is a product of Utah!
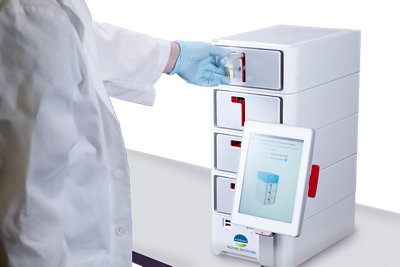
The BIOFIRE® SPOTFIRE® developped and produced in Utah, has been launched in 2023.